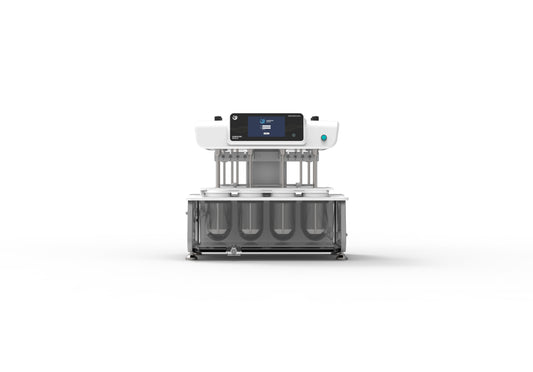
Collection: Dissolution Testing Instruments
Dissolution Testing Instruments for Tablets and Capsules
Dissolution testing is a critical step in pharmaceutical quality control and R&D. It ensures consistent drug release and bioavailability across batches. Our RCZ series Dissolution Testers are engineered to meet the latest USP, EP, and ChP standards.
Key Features of Our Dissolution Equipment
- High-precision temperature and RPM control
- Customizable baskets, paddles, and sampling systems
- Touchscreen interface with real-time data export
- Models available for 1, 6, 8, and 12 vessels
Applications
- Tablet and capsule quality testing
- Generic drug bioequivalence studies
- Research and development in pharma labs
Explore the full lineup below to find the right solution for your lab. For quotation or technical support, contact our team.
-
RCZ-1B Dissolution Tester – Pharmaceutical Testing Instrument for Tablets
Regular price $0.00Regular priceUnit price / per -
RCZ-6N Intelligent Dissolution Tester – Pharma Lab Equipment for Dissolution Testing
Regular price $0.00Regular priceUnit price / per -
RCZ-8N Intelligent Dissolution Tester – Advanced Tablet Dissolution Testing Instrument
Regular price $0.00Regular priceUnit price / per -
RCZ-8A Dissolution Tester – USP-Compliant Pharmaceutical Testing Equipment
Regular price $0.00Regular priceUnit price / per -
RCZ-12A Intelligent Pharmaceutical Dissolution Tester - Advanced Drug Dissolution Testing
Regular price $0.00Regular priceUnit price / per -
RCZ-QY8 Automatic Sampling System – Dissolution Test Automation Module
Regular price $0.00Regular priceUnit price / per -
RCZ-QY12 Automatic Sampling System – High-Throughput Dissolution Sampling Solution
Regular price $0.00Regular priceUnit price / per