Why ODF Projects Need “Equipment Power” to Scale from Lab to GMP
Share
Many oral dissolvable film (ODF) projects succeed in the laboratory stage, but struggle when transitioning to GMP-scale production. Issues such as unstable films, inconsistent content, drying failures, and sudden drops in yield are common. One root cause is the technology gap between lab-scale and production-scale equipment. To achieve a smooth R&D-to-GMP transition, strong “equipment power” is needed to bridge the gap.
Common “R&D Disconnection” Problems
- Lab coating methods differ from production-scale machines, making parameters invalid
- Drying, tension control, and liquid supply systems are not aligned with actual production
- Lack of in-line detection systems, leading to non-traceable and non-auditable data
- Lab environments often fail to meet GMP cleanliness standards, limiting regulatory support
How Equipment Supports the Lab-to-GMP Transition
- Adopt pilot equipment such as MJ150-L, designed with the same principles as GMP machines, to validate scale-up processes in advance
- Equip systems with in-line thickness measurement, automatic tension control, and closed liquid supply for consistent data
- Enable data collection and export functions to meet audit trail requirements
- Record complete process parameters to support registration and export filing
Recommended Equipment:
HUANGHAI’s Lab-to-GMP Solutions
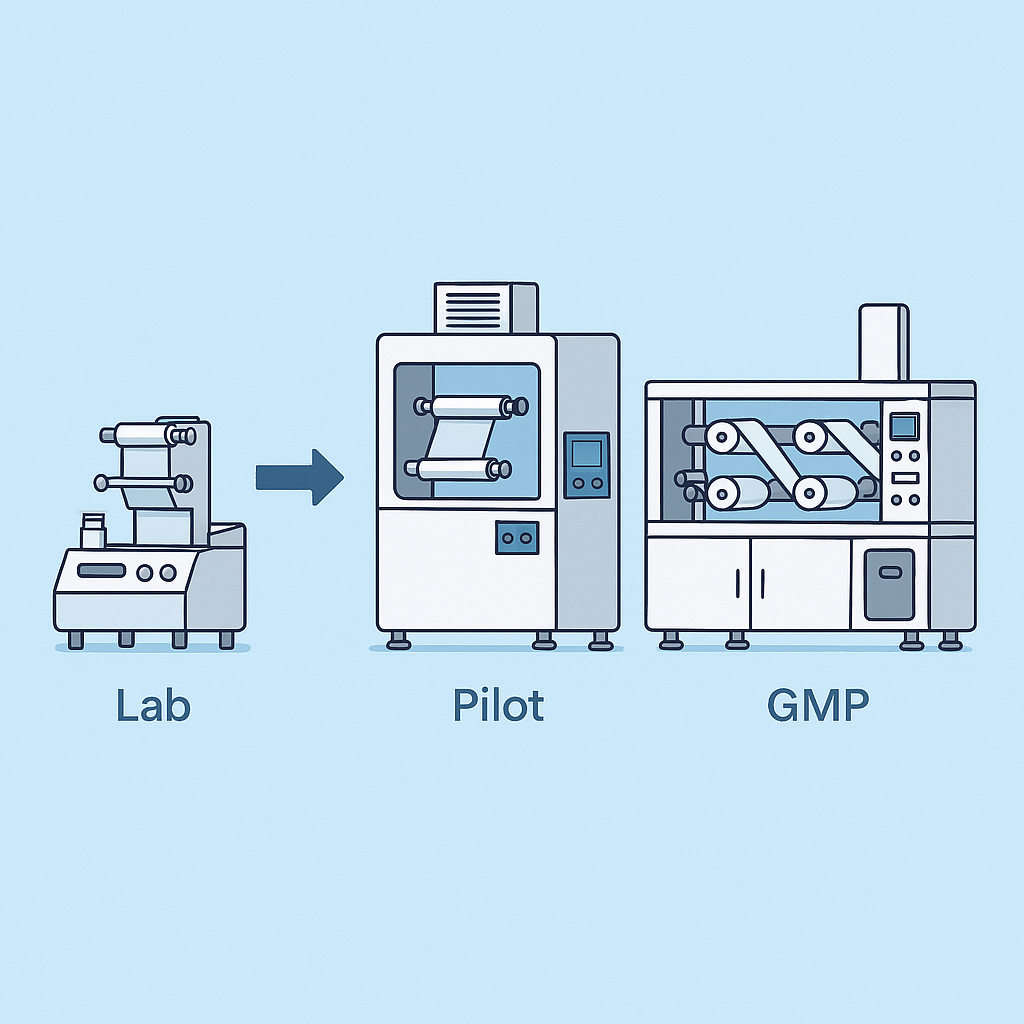
HUANGHAI offers a complete equipment ecosystem covering R&D, pilot, and commercial GMP stages:
- Lab-scale: MJ100 small coating lab system (optional)
- Pilot-scale: MJ150-L, enabling reproducible parameter migration for scale-up
- Commercial GMP: MJ150 with output up to 20,000 strips/hour, fully GMP-compliant
- Seamless integration with testing instruments, enabling a “manufacturing + testing” traceability loop
Conclusion
Success in ODF projects is not only about formulation — it is about process control and equipment alignment. Deploying pharmacopeia-grade pilot equipment and scale-up strategies early is the key to efficient market entry and successful regulatory approval.
