Why Laser Drilling Is Essential for Osmotic Tablet Controlled Release
Share
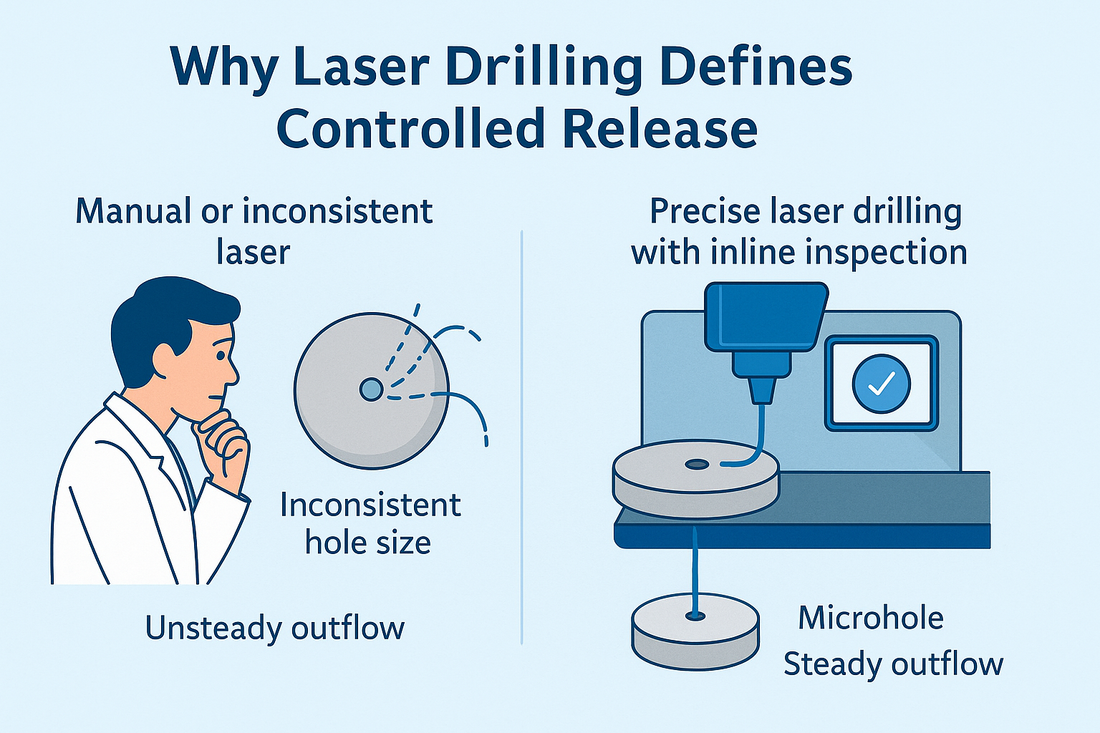
Introduction: Osmotic controlled-release tablets rely on a pressure-driven mechanism: water enters through a semi-permeable membrane, generating osmotic pressure that pushes the drug solution out through a microhole at a constant rate. This microhole acts as the “valve” of the system—without precise, reproducible drilling, the release curve cannot be reliably locked. As more chronic therapies for hypertension, prostate conditions, and similar indications shift toward long-acting osmotic formulations, the demand for hole precision and repeatability has never been higher.
Key Pain Points
- Hole diameter drift affects release rate: Smaller holes cause under-release, while oversized holes lead to dose dumping. Wide variability within a batch raises BE and consistency risks.
- Hole position deviation affects outflow stability: Off-center or edge-close holes may cause membrane defects, cracking, or premature failure.
- Single vs. dual hole trade-off: Dual holes can reduce blockage risk but increase inspection complexity and production cycle time.
- Detection and traceability challenges: “Drilling accurately” isn’t enough—compliance requires “visible and traceable.” Each hole’s presence, diameter, position, and defect image must be electronically recorded for audit readiness.
- Scale-up disconnect: From lab to production, parameters like laser power, scan trajectory, transport speed, and dust extraction vacuum must be harmonized. Otherwise, the release profile validated in R&D may fail to reproduce in manufacturing.
HUANGHAI’s Integrated Solution
The K3-2 Pharmaceutical Laser Drilling System combines drilling, visual inspection, rejection, and data storage into one platform:
- Precision laser control: Consistent energy and spot stability ensure tight hole-diameter distribution.
- Inline vision detection: Automatically identifies missing, blocked, or deformed holes with image archiving for each batch.
- Automated rejection: Defective tablets are ejected in real time without interrupting the main line.
- Data traceability: Each operation is logged and exportable, supporting full compliance with GMP and 21 CFR Part 11 requirements.
Process Optimization and Scale-Up
By correlating the target release curve ↔ hole-diameter window ↔ inline detection capabilities, teams can accelerate validation and minimize trial cycles. Stable hole geometry ensures the same release behavior from pilot batches to commercial production.
Key Takeaway
Stable, repeatable microhole geometry is essential for predictable osmotic tablet performance. HUANGHAI’s K3-2 system transforms “drilling precisely” into “drilling reliably,” linking mechanical precision with data integrity and GMP-compliant traceability—ensuring every batch meets controlled-release expectations.
