From “Accurate” to “Reliable”: Engineering Essentials for Vision Inspection and Rejection
Share
Introduction: With stricter regulatory oversight and audits, inspection and rejection determine whether drilling quality is provable and traceable. A vision system is not “just taking a photo.” It is an engineering stack that must be deeply coupled with conveyor takt, rejection execution, and data retention.
Common pain points
- Incomplete metrics: Checking only “hole present/absent” while ignoring diameter, position deviation, chipping/ablation.
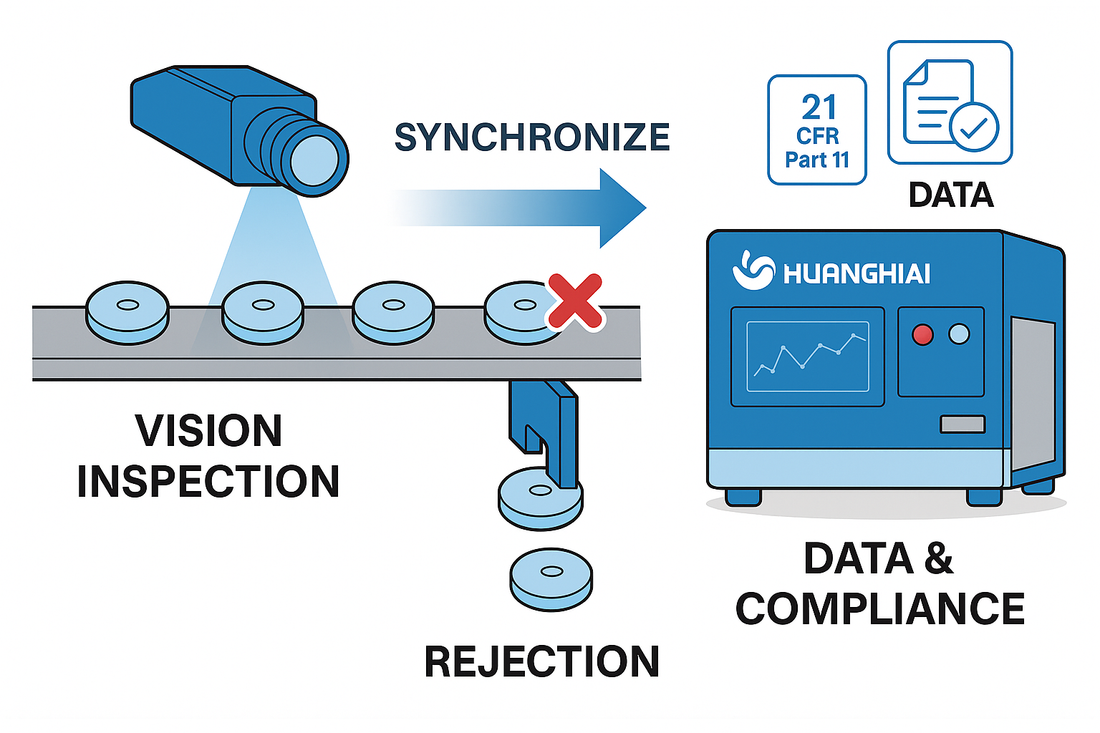
- Poor takt matching: Camera frame rate, exposure, and lighting not synchronized—causing misses or false positives.
- Inaccurate rejection: No per-tablet tracking, leading to misaligned rejection or secondary contamination.
- Unstructured data: Images and logs are merely stored, scattered, and not structured—making audits and deviation investigations slow.
HUANGHAI’s approach (K3-2 vision + rejection)
- Vision design: Lighting and lens selection tailored to tablet type/hole location; coverage for presence, diameter, and position; threshold/statistical models with parameterized management.
- Takt & synchronization: Encoder-triggered capture; calibrated time windows across camera–PLC–reject gate to ensure accurate localization at high speed.
- Rejection strategy: Per-tablet tracking ID with self-check for mis-rejection; configurable re-inspection/sampling workflows.
- Data & compliance: Automatic batch reports (CQA/CIP), alarms and disposition logs; role permissions, audit trail, and e-signature to meet 21 CFR Part 11.
- Maintainability: Modular lighting, quick lens calibration; grayscale/size check pieces and a routine verification checklist.
These engineering principles are implemented in HUANGHAI’s Olando K3-2 Pharmaceutical Laser Drilling System, which integrates high-speed vision inspection, real-time rejection, and regulatory-grade data capture within a single automation framework.
Conclusion
Upgrading “inspection” into a quality-control chain is how “accurate drilling” becomes reliable drilling. Define inspection dimensions and release criteria in the URS phase, and map them to the camera/lighting/rejection/data configuration list to ensure robust execution and audit readiness.
📘 Learn more about our integrated laser drilling solutions here: Olando K3-2 Pharmaceutical Laser Drilling System.
