Configuring Pharma-Grade ODF Production Lines: From Liquid to Finished Film
Share
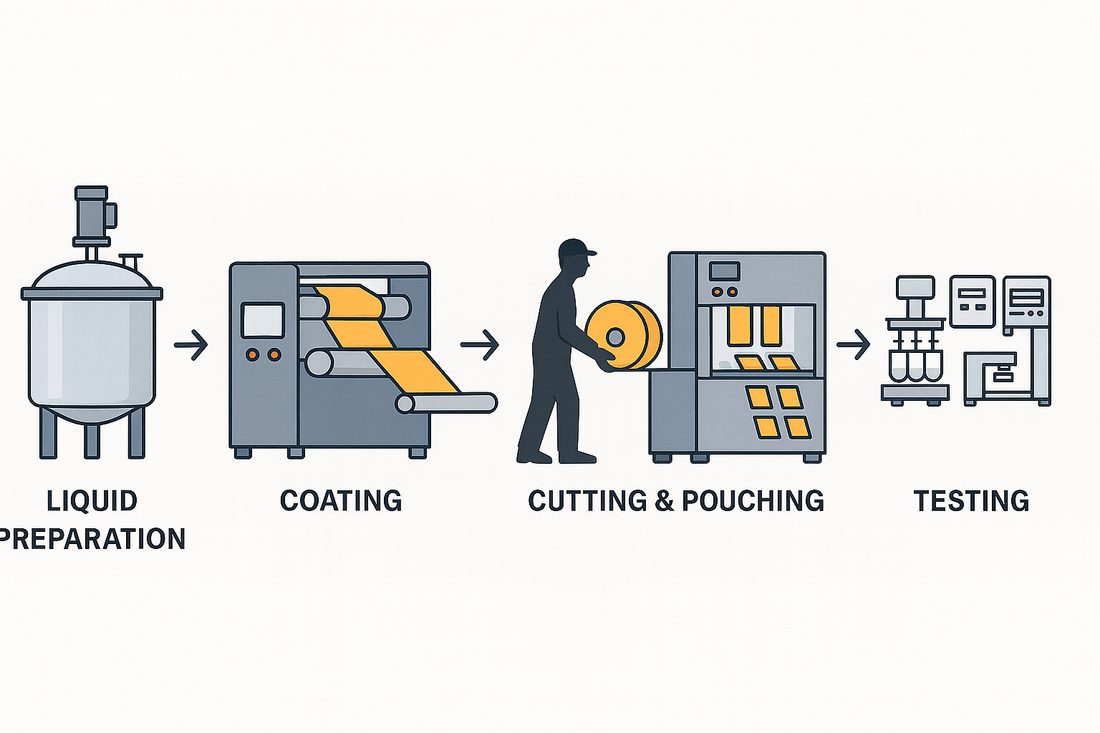
Entering the pharmaceutical market with oral dissolvable films (ODFs) requires more than GMP certification. It demands a closed-loop production system that controls every step—from liquid preparation to film coating, cutting and packaging, and integrated quality testing. This is not simply a set of machines, but a system-level approach to quality, efficiency, and regulatory responsibility.
1) Why Is End-to-End Control Critical for Pharma-Grade ODF?
ODFs differ from conventional dosage forms: they are thin, micro-dosed, and highly sensitive to formulation distribution. Regulatory authorities scrutinize every detail during drug registration, including:
- Traceability of formulation uniformity
- Batch-to-batch consistency of thickness and content
- Sealing integrity and clear identification of packaging
- Complete data records and audit-ready documentation
Only a closed-loop system ensures compliance with these stringent requirements and smooth market entry.
2) Recommended Pharma-Grade ODF Line Configuration
1) Liquid Preparation System
Advanced Pharmaceutical Manufacturing Solutions
- Pharma-grade stainless steel with fully enclosed mixing
- Inline temperature and pH control
- Optional CIP/SIP cleaning modules
- Traceable records of formulation ratios and operations
2) MJ150 Coating System
- Slot-die coating with ±0.02 mm thickness accuracy
- Hot-air gradient drying with automatic tension adjustment
- Full parameter recording: temperature, humidity, speed, tension
- Integration with inline thickness measurement for real-time feedback
3) MJF180 Cutting & Packaging System
MJF180 ODF Cutting & Packaging Machine
- Compatible with aluminum foil, PE films, and multilayer laminates
- Multi-channel cutting, coding, and batch traceability
- Expandable inline weight checking and vision inspection
- Compliant with international packaging traceability standards
4) Integrated Testing Instruments
Pharmaceutical Testing Instruments
- Dissolution, disintegration, thickness, and content uniformity testing
- Data export aligned with FDA 21 CFR Part 11 audit requirements
- Real-time QC platform supporting both production and registration submissions
3) Applications for Pharma-Grade ODF Lines
- OTC drugs: Cough relief, allergy treatment, oral pain relief
- Pediatric and geriatric formulations: Vitamin D, B-complex, sleep aids
- Prescription drugs: High-value formulations with unique absorption pathways
- Export & regulatory projects: Sample production and data packages for EU/US market submissions
Conclusion
ODFs are not just “films”—they are advanced drug delivery systems. A pharma-grade ODF line must integrate liquid prep, coating, packaging, and testing into a closed-loop framework. This ensures GMP compliance, registration-ready data, and global market access. With closed-loop technology, data integrity, and transparent processes, pharma manufacturers can confidently scale ODF innovations into commercial reality.
