Olando K3-2 vs Other Brands: The Practical Choice for GMP Lines
Share
When selecting a laser tablet drilling system for osmotic tablets (e.g., Metformin, Nifedipine), real-world performance beats brochure numbers. This article compares our Olando K3-2 with other international brand solutions from the angles that matter most on a GMP floor: operation, cleaning, throughput, compliance, total cost, and service response.
Why this comparison matters
- More than peak speed: Operators, cleaning/changeover, compliance, and response time drive OEE and time-to-validation.
- GMP realities: Space is tight; digital records must pass audits. A compact, Part 11–ready system starts up faster.
- ROI focus: If your target is up to 100k–120k tablets/hour, the right balance of usability and cost wins.
Key Differences at a Glance
| Dimension | Olando K3-2 | Other International Brands | What it means on the line |
|---|---|---|---|
| Operation | Simple HMI, quick parameter recall; fast start/stop | Often more steps and deeper menus | Shorter training & fewer operator errors |
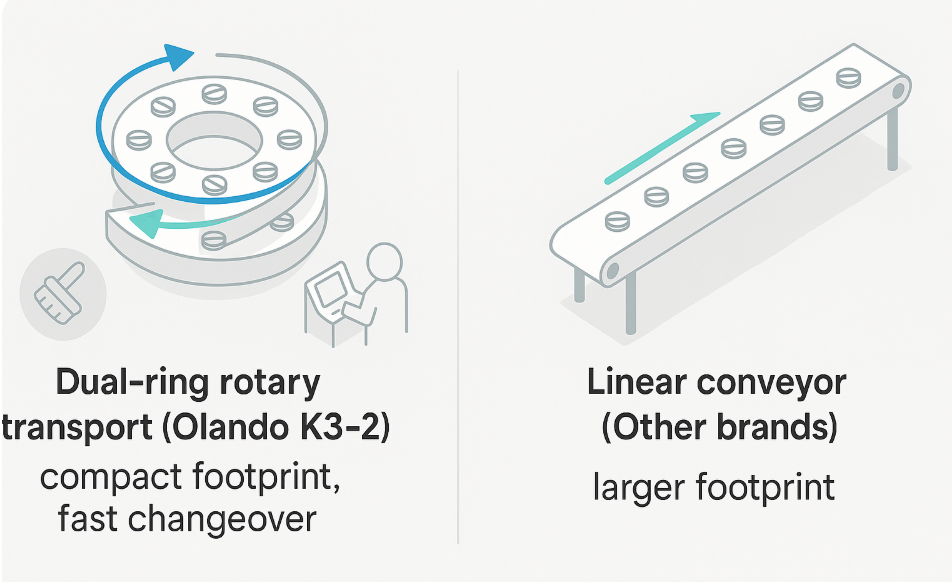
| Cleaning & Changeover | Dual-ring rotary transport, compact path; easier daily cleaning & batch change | Linear conveyor & longer footprint mean larger cleaning coverage | Faster changeover = higher effective throughput (OEE) |
| Throughput | Up to 120,000 tablets/hour | Some brands advertise up to ~140,000/h | Both cover 100k–120k/h targets; others may edge higher on peak |
| Compliance | 21 CFR Part 11–ready: user access, e-signatures, audit trail; IQ/OQ documentation available | Typically similar claims | Audit-ready data integrity for regulated markets |
| Footprint | Compact layout thanks to dual-ring transport | Longer linear lines | Better space utilization in GMP rooms |
| Total Cost & ROI | More competitive overall budget at comparable configurations; target ≤ 3-month delivery with on-site install & training | Higher overall investment is common | Faster ramp-up and payback |
| Service Response | < 24-hour response (remote/on-site coordination) | Often longer response times (customers commonly report ~2 days) | Lower downtime risk at a critical process node |
What both classes can do
- Dual-camera inspection with reject mechanisms for defective tablets.
- GMP-aligned digital records and support for 21 CFR Part 11 features.
- Commercial-scale deployment in osmotic tablet production.
Selection guidance
- Space-constrained GMP rooms, fast start-up, and easy maintenance: K3-2’s compact, dual-ring design and quick cleaning/changeover are highly advantageous.
- Pursuing absolute peak speed: Some international brands may publish a slightly higher ceiling; weigh footprint, budget, and response time trade-offs.
- Audit-led projects: Request Part 11 features (user roles, e-signatures, audit trail) and IQ/OQ templates from any vendor—K3-2 provides both.
Engineering highlights (K3-2)
- Transport: Dual-ring rotary tablet transport for compact footprint and stable high throughput.
- Vision & Lighting: Cognex vision, CCS lighting; real-time inspection and reject.
- Control: Linux-based upper system; PLC options include Panasonic or Siemens upon request.
- Compliance: 21 CFR Part 11 support (user access control, electronic signatures, audit trail); IQ/OQ documentation available.
- Support: On-site international installation and 1-week training; < 24-hour support response.
Procurement & validation checklist
- Part 11: user roles, e-signature, audit trail; sample logs/screenshots.
- IQ/OQ package readiness; SOPs and training materials.
- Cleaning/changeover: dead-spot access, typical time for size/recipe change.
- Throughput target: stable 100k–120k/h or chasing higher peak?
- MES/EBR connectivity and export formats for traceability.
- Service SLA: response time, spare parts lead time, preventive maintenance plan.
See the machine
Product page: Olando K3-2 Pharmaceutical Laser Drilling System
Conclusion
If your goal is a validated, stable 100k–120k/h line with faster start-up, the combination of simpler operation, easier cleaning/changeover, compact footprint, Part 11 readiness, and a more competitive overall budget makes K3-2 a practical choice. Add the < 24-hour response time, and your risk of prolonged downtime at this critical process node goes down significantly.
Ask us for a one-page comparison sheet, audit-trail samples, and a cleaning/changeover checklist tailored to your product format.
