From URS to PQ: Laser Drilling Validation Path with Part 11 Readiness
Share
Introduction
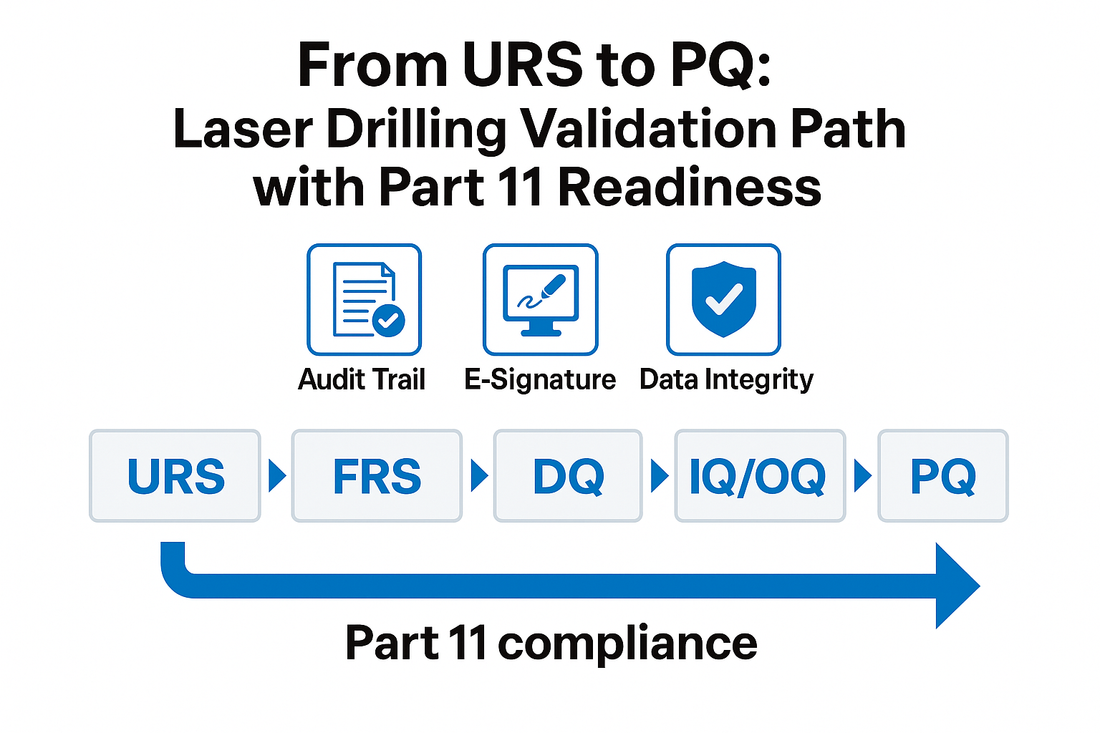
As regulators tighten data integrity requirements, validation of laser drilling systems must ensure complete traceability from design intent to operational performance. Parameters, inspection images, and rejection logs must be captured consistently across URS → FRS → DQ/IQ/OQ/PQ, in line with FDA 21 CFR Part 11 expectations.
1. Common Challenges in Validation
- Incomplete documentation loop: URS does not align with final delivery, causing audit gaps in design traceability.
- Non-compliant electronic records: Missing user permissions, audit trails, or electronic signatures; unclear backup/retention strategy.
- Opaque interfaces: MES/ERP integration fields not clearly defined, leading to repeated validation cycles.
- Insufficient training: Lack of deviation handling, change control, and re-validation trigger criteria.
2. HUANGHAI’s Validation & Compliance Support
- Documentation System: Provides FRS/FDS, risk assessment, test protocols, and record templates based on customer URS — covering drilling, vision, rejection, and data workflows.
- Part 11 Essentials: Implements user-level permissions, password policy, audit trail, e-signature, time sync, data backup & recovery; offers data export and retention guidelines.
- Interface Specification: Supplies MES/ERP data dictionary & event tables (batch start/end, parameter set, alarm, rejection statistics, image index, etc.).
- Training & Handover: On-site/remote training, preventive maintenance plans, change control, and re-validation trigger lists to ensure sustainable compliance.
3. Summary
Include data and system interfaces in your validation plan from the start. Use a risk-based approach to define test depth. HUANGHAI supports documentation, functionality, interfaces, and training — enabling validation that is both compliant and efficient.
🔗 Related Product: Olando K3-2 Pharmaceutical Laser Drilling System
