GMP Cleanliness & Dust Extraction: Hidden Essentials at the Laser-Drilling Station
Share
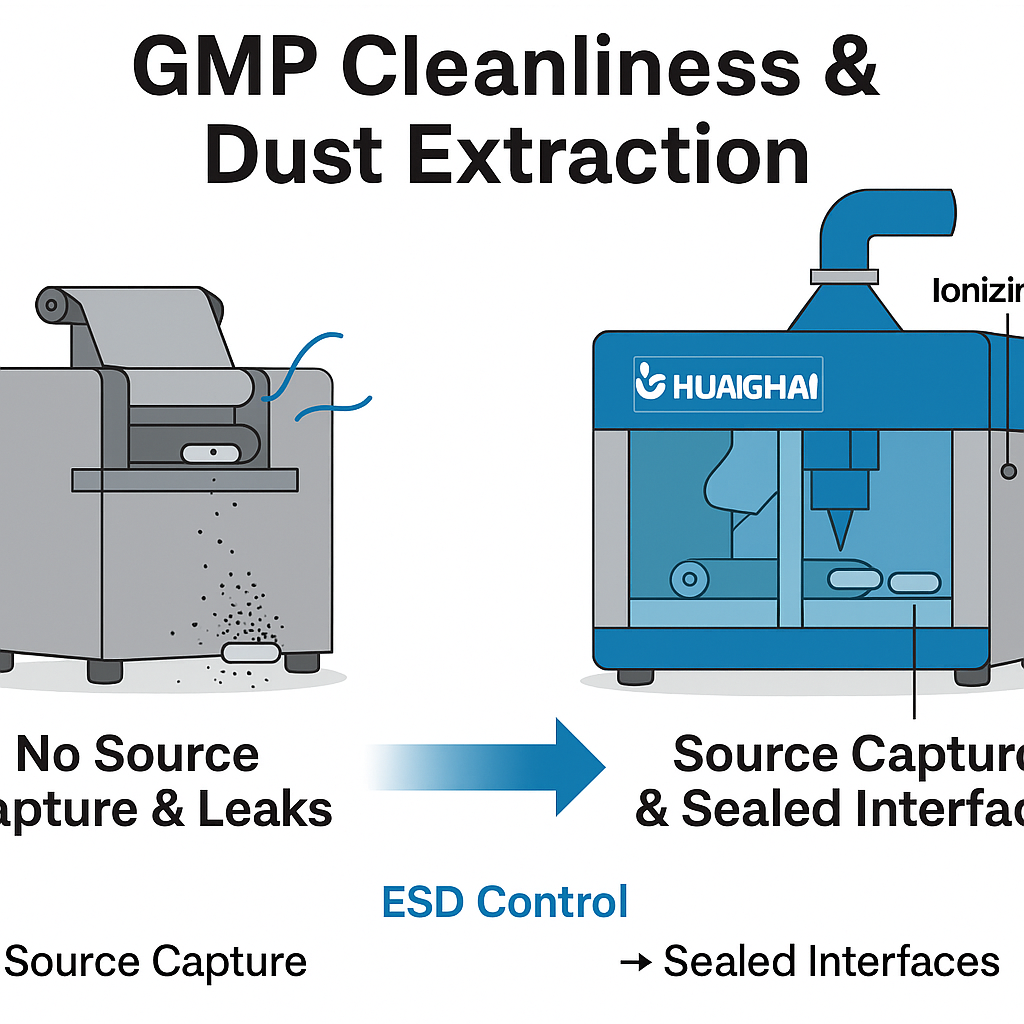
Introduction: Laser processing unavoidably creates fine ablation debris. If dust control is inadequate, consequences include poor hole edges, cross-contamination, equipment buildup, and difficult cleaning validation. In a GMP facility, cleanliness must be engineered from the source—not left to accessories.
Pain points
- Dust fallback: Residue around the hole rim affects downstream inspection and increases false rejects.
- Insufficient enclosure sealing: Risk of secondary contamination in the room or loss of designed negative pressure.
- Complex cleaning validation: Hard-to-remove contact parts and dead zones make SOPs difficult to execute.
-
Static and carry-over: Static buildup during conveyance/collection causes dust adhesion and transfer.

HUANGHAI’s integrated approach (equipment + process)
- Source capture: Local high-velocity extraction at the laser area with adjustable negative pressure; ductwork and filtration levels configured to the target cleanroom grade.
- Enclosure & docking: Semi-sealed process chamber with sealed feed/discharge interfaces to minimize outward leakage.
- Contact parts & disassembly: Pharma-grade materials for tablet-contact components; quick-release structures for easy cleaning and visual inspection.
- ESD control: Ionizing air and anti-static materials at key nodes to reduce dust adhesion and carry-over.
- Validation & SOPs: Checklists and record templates for inspection/cleaning/replacement, supporting DQ/IQ/OQ/PQ and routine audits.
These measures are implemented on HUANGHAI’s laser drilling platform to protect both quality and compliance. For system details, see the Olando K3-2 Pharmaceutical Laser Drilling System.
Conclusion
Dust extraction and cleanliness are not “add-ons”; they are core engineering controls that influence quality and compliance. Define quantitative requirements in the URS—negative pressure, filtration class, disassembly/inspection time, and inspection frequency—to shorten the cleaning-validation path and de-risk audits.
