Launch Fast, Prove Consistency: New-Line Laser Drilling + MES Integration for CR Tablets
Share
Context: New controlled-release tablets live or die by the drilled hole. Teams must lock hole geometry quickly and produce audit-ready data from day one. This post outlines typical greenfield problems, our solution stack, and a recent doxazosin CR case.
Industry Problems When Building a New Line
- Parameter discovery: fast alignment of hole presence/size/position with the intended release profile.
- On-line verification: proving uniformity in real time, not by delayed sampling.
- Data integrity at go-live: clear batch records and event/alarm trails under GMP.
- Ramp-up pressure: operators need practical training; validation timelines are tight.
The Solution Architecture We Provide
- Olando K3-2 laser drilling units installed in GMP-certified cleanrooms.
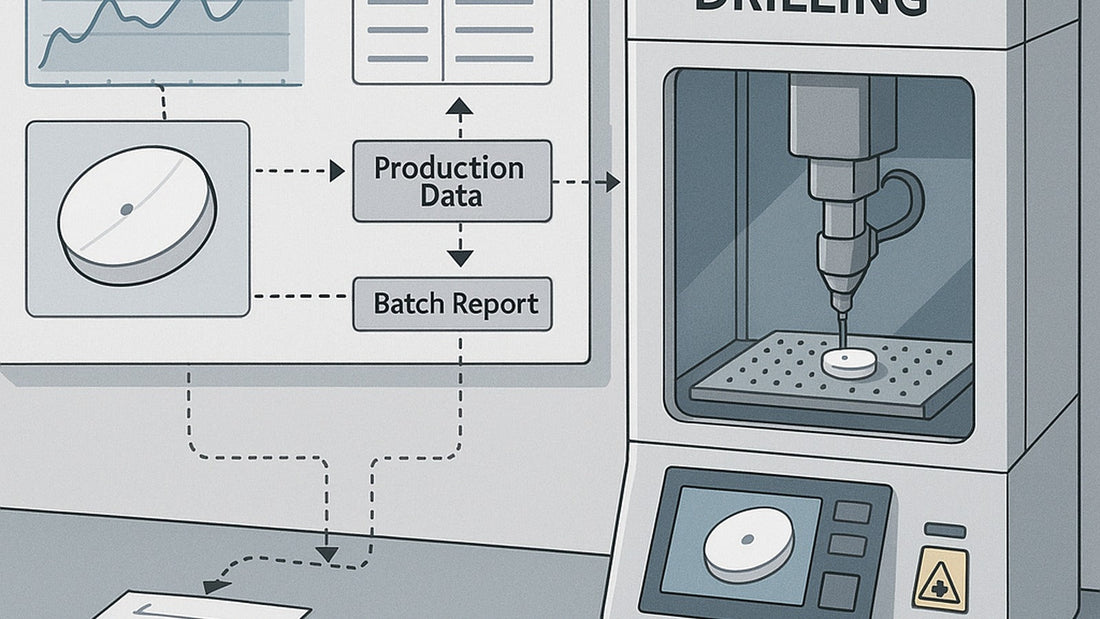
- Inline vision inspection for presence/size/position + automatic rejection.
- Electronic records with optional MES mapping (batches, events, alarms).
- Validation documents plus operator/maintenance training and remote support after go-live.
Case Evidence: Doxazosin CR, 2023 (3× K3-2 + MES)
In 2023, a pharma team commissioned three K3-2 systems for a new doxazosin controlled-release tablet project. Each system was deployed in a GMP cleanroom, connected to the plant’s MES, and configured with vision thresholds and rejection logic tied to validation. The result was faster parameter lock-in and audit-ready electronic records from the start, with a clear path to add capacity later.

Rapid-Launch Checklist
- Translate URS for hole geometry into laser parameters and vision thresholds.
- Run challenge sets to test presence/size/position boundaries.
- Agree on rejection criteria with QA; document periodic challenges.
- Enable e-records; configure MES tags for batches, alarms, and exceptions.
- Complete operator/maintenance training with shift coverage; schedule remote support windows.
Explore Olando K3-2 · See Solution Details · Request Trials or Integration Advice
