How Fully Automatic ODF Packaging Systems Boost Efficiency and Consistency
Share
As ODF (oral dissolvable film) products scale to industrial volumes and international markets, manual cutting and pouching can no longer meet requirements for packaging efficiency, product consistency, and regulatory compliance. Fully automatic packaging systems not only reduce human error and waste, but are also the key to passing GMP audits and unlocking higher throughput.
1) Common Pain Points in ODF Packaging
- Uneven edges and size variation: Manual cutting leads to higher defect rates.
- Cross-contamination risks: Film-to-pouch handling may introduce impurities and affect stability.
- No unit-level serialization: Difficult to implement batch control and digital traceability.
- Low packaging speed: Manual operations limit output for the entire line.
- Poor batch yield: Lack of inline inspection and rejection keeps the good-rate unstable.
2) Highlights of a Fully Automatic ODF Packaging System
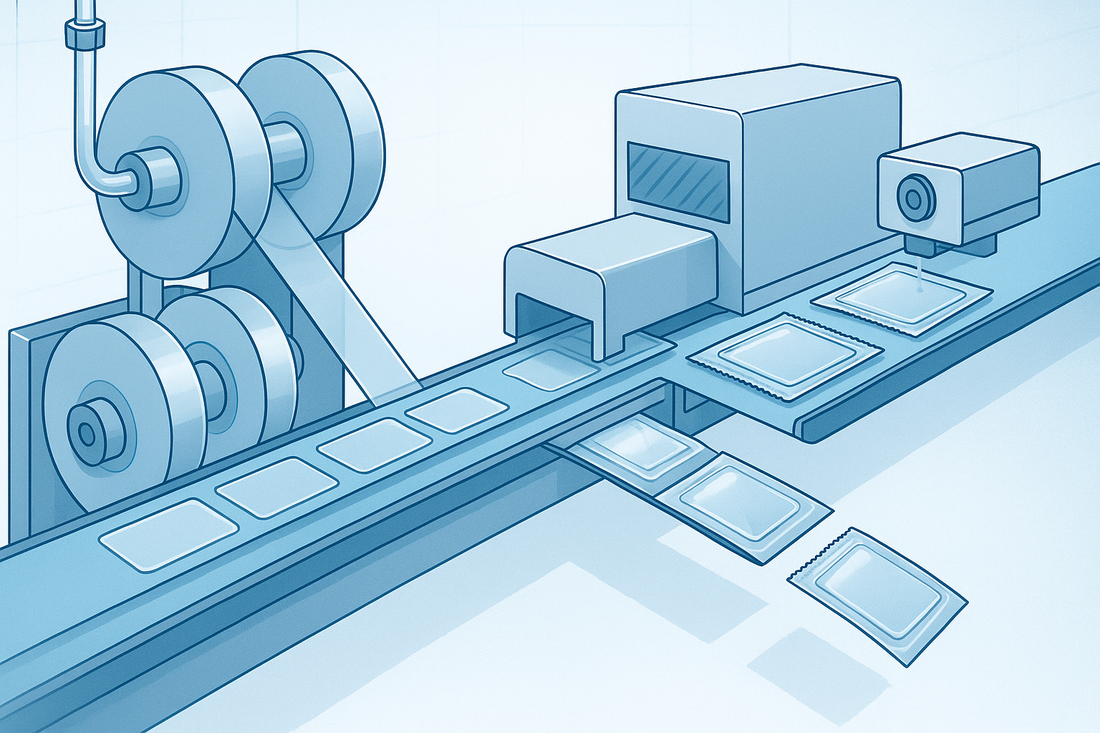
A modern ODF packaging line integrates cutting, sealing, coding, inspection, and rejection into one continuous workflow to significantly increase efficiency and batch-to-batch consistency.
MJF180 High-Speed Strip Cutting & Packaging
- Dose-accurate cutting: Fixed-length with registration tracking for precise unit size.
- Dual-lane high throughput: Output up to 20,000 strips/hour (process- and material-dependent).
- Clean sealing options: Pneumatic or heat-seal to ensure intact, contamination-free edges.
- Inline coding & serialization: Laser coding supports one-code-per-unit and traceability.
View the MJF180 ODF Strip Cutting & Packaging Machine
QC Integration
- Inspection linkage: Can interface with downstream systems (e.g., dissolution or content-uniformity checks) to auto-reject nonconforming units.
- Real-time data capture: Supports audit trails and continuous process oversight.
Compliance & Data
- GMP-aligned design: Enclosed structures and hygienic layouts support clean packaging.
- Data storage & export: Records can be archived and exported; architecture is designed to support FDA 21 CFR Part 11 electronic audit requirements.
- Material flexibility: Compatible with paper pouches, aluminum foil, and multilayer laminates.
3) Where Fully Automatic Packaging Delivers the Most Value
- CDMO and export programs: Meet international standards for packaging uniformity.
- Drug-loaded film packaging: High demands on hygiene and seal integrity.
- High-throughput ODF plants: Increase line output while reducing labor costs.
- Serialization projects: Enable unit-level traceability for sampling, recalls, and regulatory submissions.
Conclusion
Success in ODF manufacturing depends as much on packaging as on film-making. Choosing a fully automatic system with precise cutting, clean sealing, and digital traceability translates into higher yield, faster market response, and stronger global compliance. The MJF180 platform brings these capabilities together to raise both productivity and assurance.
